Susvimo
also known as Ranibizumab Ocular Implant
Last updated August 26, 2025
Medical information on this page is for educational purposes only and is not a substitute for professional medical advice, diagnosis or treatment.
See our Terms & Conditions and Consent for Telemedicine for details.
Overview
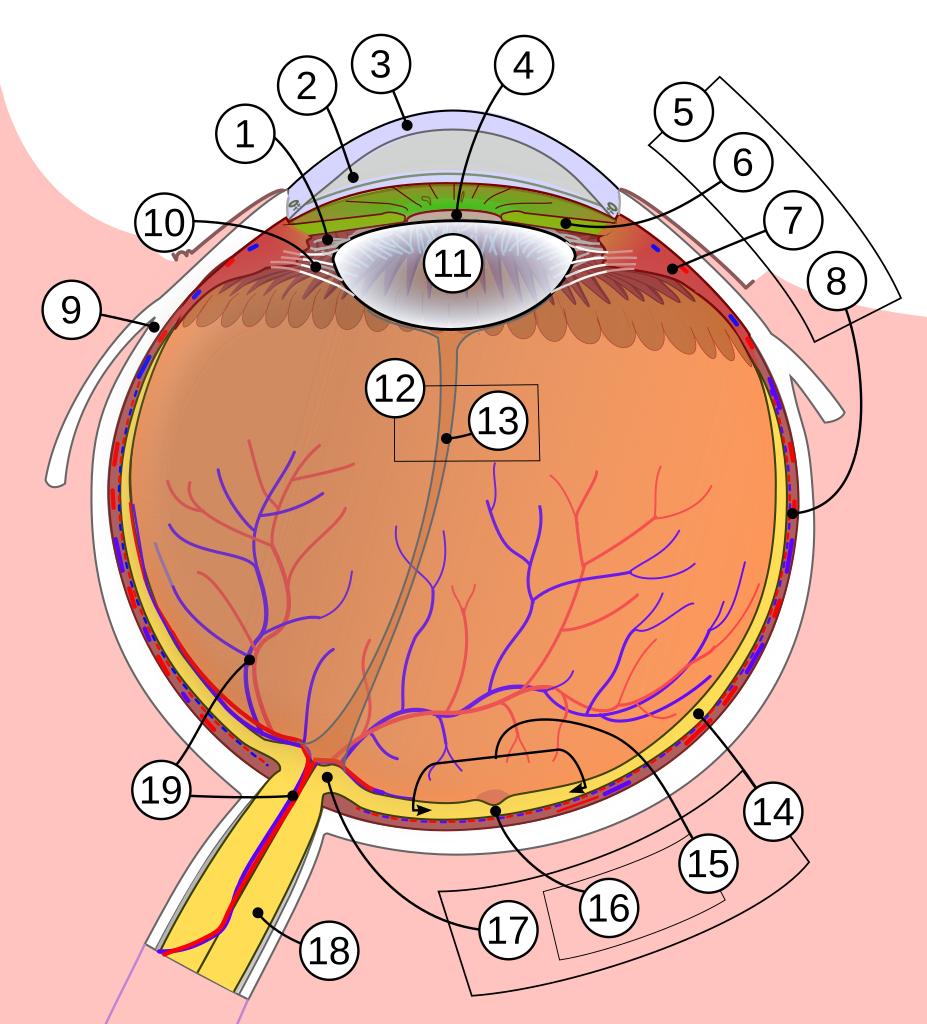
Susvimo is a small, refillable implant that slowly releases ranibizumab (an anti-VEGF medicine) into the eye to treat conditions where leaky blood vessels harm the macula, such as neovascular (wet) age-related macular degeneration (AMD). These conditions can blur central vision and make straight lines look wavy.1
According to the U.S. prescribing information, Susvimo is indicated for adults with wet AMD, diabetic macular edema (DME), and diabetic retinopathy (DR) who have already responded to at least two prior anti-VEGF injections. Typical refill schedules are every 24 weeks for AMD and DME and every 36 weeks for DR.2
How the Procedure Works & Options
What happens:
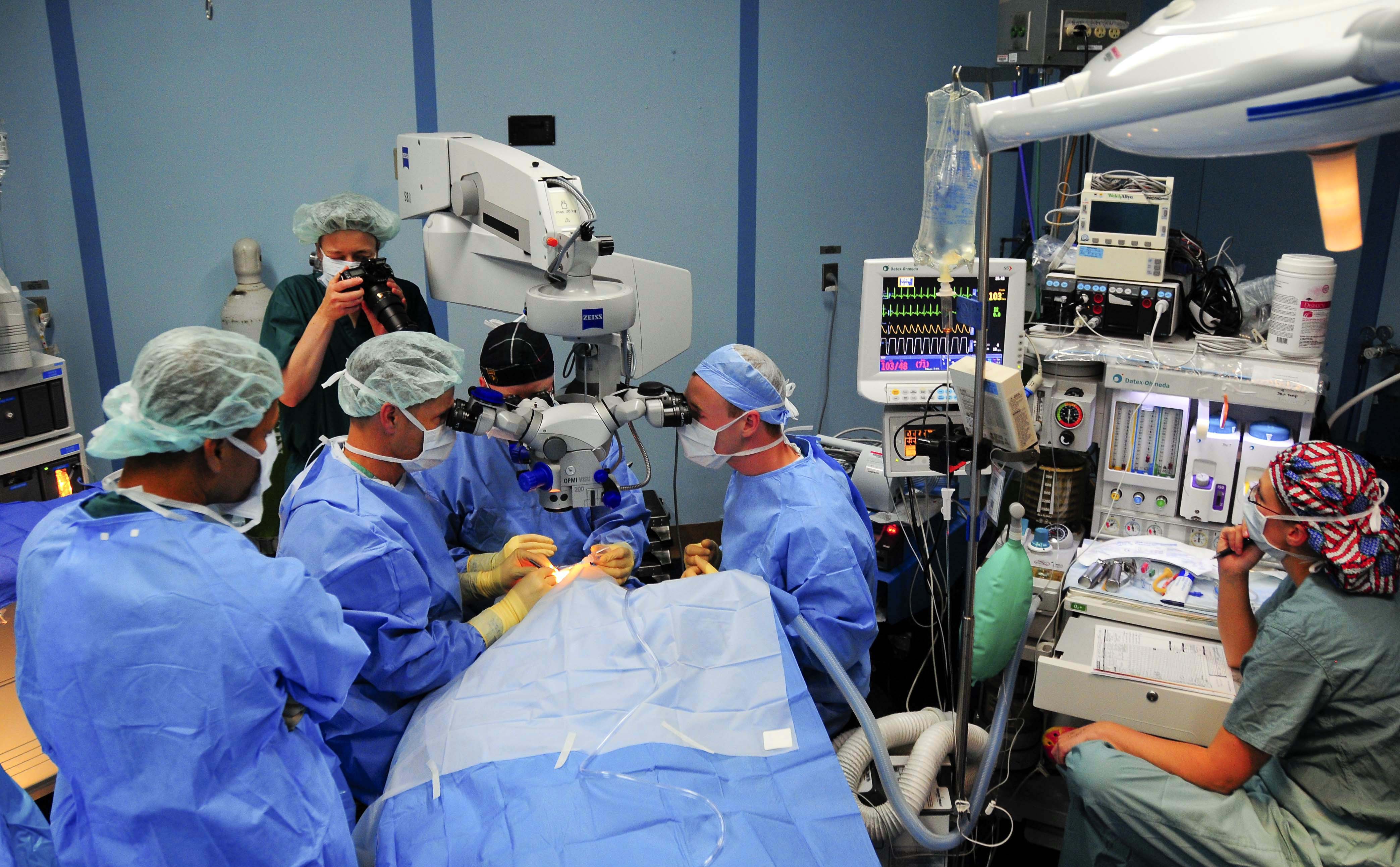
- Implantation: A retina surgeon places the tiny reservoir in the white part of the eye (sclera) in an operating room. It is filled with ranibizumab and releases medicine continuously.
- Refill-exchange visits: The old medicine is exchanged for fresh medicine through the implant’s port under numbing drops with sterile technique, typically every 6 months for AMD/DME and every 9 months for DR.
- Removal: If needed, the implant can be surgically removed.
These steps, intervals, and sterile-procedure requirements are specified in the FDA-approved labeling.3 An alternative to Susvimo is continuing standard office-based anti-VEGF injections (such as ranibizumab, aflibercept, or faricimab) on a schedule your retina specialist sets.4
Who Is a Candidate?
Good candidates usually include adults with wet AMD, DME, or DR who have already shown a helpful response to at least two prior anti-VEGF injections and who prefer fewer injection visits.5
People should not get the implant if they have:
- An active eye or eyelid infection
- Active inflammation inside the eye
- A known allergy to ranibizumab or implant components
Your surgeon will also check your ability to attend follow-up and refill visits and the health of the conjunctiva (surface tissues) where the device sits.
Enter your details below to check your suitability for this treatment
Suitability Level
Recommendation
Cost and Price
Costs include:
- The medicine
- Surgery to place the implant
- Later refill-exchange visits
In the U.S., drugs given by a clinician in an office or outpatient setting are often covered under Medicare Part B (or other insurance) when medically necessary, though deductibles and coinsurance may apply.7
Exact out-of-pocket costs vary by insurance plan, location, and number of refills. CMS notes Part B drug coverage typically requires that the drug is furnished incident to a doctor’s service; patients are responsible for the Part B deductible and usually 20% coinsurance unless supplemental coverage applies.8
Benefits and Limitations
Benefits: In the phase 3 ARCHWAY trial for wet AMD, vision with Susvimo refilled every 24 weeks was statistically non-inferior to monthly ranibizumab injections through two years, while greatly reducing injection frequency.9 Many patients value fewer clinic shots and the convenience of planned refill visits.14
Limitations & risks: Because Susvimo involves a surgical implant, there is a higher risk of certain complications compared with injections alone, including a higher rate of endophthalmitis (serious eye infection). Risks also include retinal detachment, vitreous hemorrhage, conjunctival erosion or retraction, and implant displacement.10
Recovery and Long-Term Care
After surgery, your eye may feel scratchy, light-sensitive, or slightly blurry. A temporary drop in vision is common over the first two postoperative months. You will use prescribed drops and return for checks to be sure the conjunctiva heals well over the implant.11
Call your retina specialist right away for:
- Increasing pain
- Worsening redness
- Pus
- Sudden vision loss
- New flashes or many new floaters
These can be signs of urgent problems. Professional societies emphasize sterile technique and prompt care for warning symptoms.12
Latest Research & Innovations
Long-term safety and outcomes are being followed in the PORTAL extension study of patients who completed LADDER or ARCHWAY. Interim reports continue to characterize adverse events and durability over extended follow-up.13
Patient-reported outcomes also matter. A randomized study in JAMA Ophthalmology found high satisfaction overall, with most patients assigned to the port delivery system preferring it to frequent injections at week 40.14
Recently Published in Peer-Reviewed Journals
Ophthalmology. Retina
February 1, 2025
Endophthalmitis in Eyes Treated with the Port Delivery System with Ranibizumab: Summary of Cases during Clinical Trial Development.
Eichenbaum DA, Freeman WR, Chang MA, et al.
Ophthalmology. Retina
May 1, 2024
Cost-Utility Analysis of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration.
Brown GC, Brown MM, Monigle MC
Ophthalmology. Retina
February 1, 2024
Exuberant Fibrosis over a Susvimo Implant.
Bylund R, Nielsen JS, Ericksen C
Next Steps
If you think Susvimo could help you, schedule a visit with a retina specialist (ophthalmologist) to review your diagnosis, prior response to anti-VEGF therapy, surgical candidacy, and follow-up needs. You can search the American Academy of Ophthalmology’s directory to find an eye M.D. near you.15
Important: If you have sudden vision changes, severe eye pain, new flashes, or many floaters, seek urgent care immediately.16
Kerbside can help you connect with the right specialist for an education-focused consult about Susvimo and other options (this is not a physician–patient relationship or medical care).
Trusted Providers for Susvimo

Dr. Emily Eton
Specialty
Retina/Vitreous
Education
Harvard Medical School

Dr. Grayson Armstrong
Specialty
Retina/Vitreous
Education
Ophthalmology

Dr. Jose Davila
Specialty
Retina/Vitreous
Education
Retina/Vitreous Surgery

Dr. Nicholas Carducci
Specialty
Retina/Vitreous
Education
University of Pennsylvania Perelman School of Medicine