Durysta
Also known as Bimatoprost Intracameral Implant
Medical Disclaimer: Information on this page is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment.
See our Terms and Telemedicine Consent for details.

Overview
Durysta is a tiny, dissolvable implant that slowly releases bimatoprost—a proven glaucoma medicine—inside the front of the eye. It is used to lower eye pressure (intraocular pressure, or IOP) in adults with open-angle glaucoma (OAG) or ocular hypertension (OHT). Lowering IOP helps protect the optic nerve and reduces the risk of vision loss over time.1
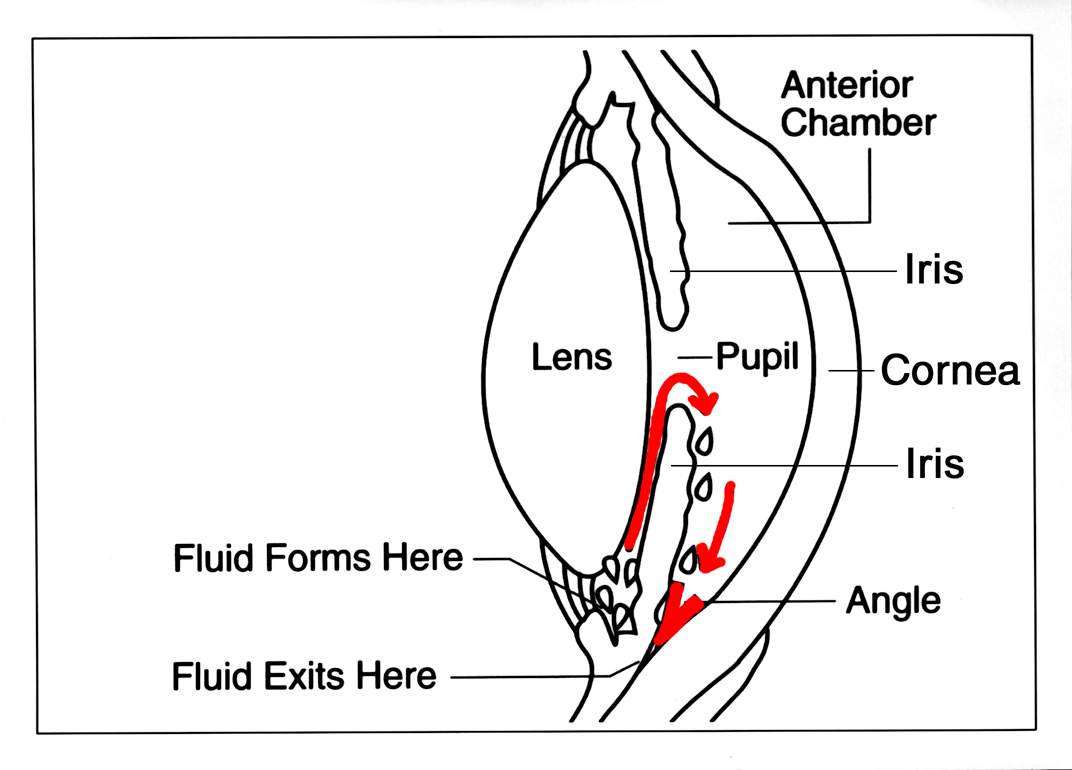
The implant sits in the lower angle of the eye and delivers medicine over weeks to months, avoiding the need to remember daily drops. Durysta is designed for a single use per eye because repeated implants may increase the risk of corneal cell loss, as explained in the approved prescribing information.2
How the Procedure Works & Options
What happens on the day? After numbing the eye and cleaning the surface, your ophthalmologist uses a preloaded, single-use applicator to gently place the Durysta implant into the anterior chamber (the fluid-filled space in front of the iris). The implant is intended to settle in the inferior angle, where it dissolves while releasing medication. The medicine lowers pressure by increasing fluid outflow, similar to prostaglandin eye drops, but without daily dosing.2
Durysta is one of several sustained-release approaches being studied and used for glaucoma; other long-acting delivery systems and depot therapies are described in professional reviews of sustained-release glaucoma treatments.3
How it fits with other options: Your overall plan may include drops, in-office laser (such as trabeculoplasty), minimally invasive glaucoma surgery (MIGS), or traditional surgery. Durysta offers an alternative for people who struggle with drops or need steadier pressure control; your surgeon will help decide if it adds value versus other treatments for your stage of disease.
Who Is a Candidate?
Typically considered for: Adults with open-angle glaucoma or ocular hypertension who need better pressure control or who have trouble using daily drops. The U.S. labeling describes intracameral use with a single implant per eye, placed under clean, aseptic conditions by a trained ophthalmologist. Expert summaries from the American Academy of Ophthalmology (AAO) also discuss how the implant can fit into modern glaucoma care for appropriate patients.4
Who should not receive it? People with conditions listed in the product labeling—such as corneal endothelial cell dystrophy (e.g., Fuchs’), a prior corneal transplant, or a ruptured/absent posterior lens capsule—should not receive Durysta, and the implant should be avoided in eyes with very narrow angles. Decisions also consider guideline-based glaucoma care, including disease severity and target pressure goals outlined in the AAO Preferred Practice Pattern for POAG.9
Is Durysta (Bimatoprost Implant) Right for Me?
Select your details to estimate suitability.
Cost and Price
How it’s billed: In the U.S., Durysta is a physician-administered drug that is typically provided and implanted in a clinic or surgery center ("buy-and-bill"). When medically necessary, Medicare Part B and many commercial plans cover certain outpatient drugs given by a clinician; your costs depend on your plan’s deductible and coinsurance rules.7 CMS explains that Part B covers drugs furnished incident to a physician’s service under specific conditions (for example, injected in the office), with payment set by published methods such as Average Sales Price plus a percentage add-on.8
What you can do: Ask your doctor’s office to verify benefits, check any prior authorization, and estimate out-of-pocket costs before the procedure. If you have a Medicare Supplement or a Medicare Advantage plan, coinsurance and network rules may differ; your clinic can help you understand your share based on site of service and coverage specifics.
Benefits and Limitations
Benefits: In phase 3 randomized trials (ARTEMIS), bimatoprost implant lowered IOP comparably to standard therapy during the primary evaluation period, with many eyes maintaining reduced pressure after a single administration. These studies support the implant’s ability to control IOP while reducing reliance on daily drops in suitable patients.5 Long-acting delivery can also help people who struggle with drop schedules or have difficulty instilling drops correctly, offering steadier exposure to medication between visits.
Limitations & risks: The label warns about corneal endothelial cell loss and other corneal adverse events; therefore, use is limited to one implant per eye without retreatment, and Durysta is contraindicated in certain corneal conditions or when the lens capsule is not intact.2 Like any intraocular procedure, there are risks (e.g., inflammation, eye redness, discomfort, or pressure changes). Your surgeon will weigh potential benefits against these risks and will monitor your cornea and pressure after treatment.
Recovery and Long-Term Care
Right after the procedure: You’ll go home the same day. Mild redness, light sensitivity, or a scratchy feeling are common for a short time. The prescribing information advises standard sterile technique, instructs patients to remain upright for about an hour so the implant can settle, and emphasizes careful follow-up, especially in those with limited corneal cell reserve.11 Your care team will check vision, IOP, the cornea, and the implant position at follow-up visits, and may adjust your drop regimen as pressure responds over time.
When to call urgently: Severe pain, rapidly worsening redness, sensitivity to light, or a big change in vision are warning signs—seek prompt care since these can indicate infection or a serious pressure problem. Typical glaucoma follow-up schedules after procedures (first day, then in 1–2 weeks, then as needed) are summarized in AAO benchmarks and will be tailored to your eye.10
Latest Research & Innovations
Long-acting control: Research shows that a single bimatoprost implant can lower IOP across the 24-hour cycle at 8 weeks and support pressure control through one year in many eyes, aligning with the goal of steadier, drop-free therapy for selected patients.6 Reviews of the phase 3 program describe randomized, masked trials comparing repeated fixed-interval implants with standard therapy, helping clinicians understand the efficacy window and safety considerations of dosing strategies.13
Evolving role in care: Additional peer-reviewed studies continue to examine how intracameral bimatoprost compares with other pressure-lowering options and when it fits best in the treatment pathway—especially for people with difficulty using drops or with ocular surface issues. New comparative and mechanistic work (including studies versus selective laser trabeculoplasty) adds context for shared decision-making about durability, safety, and patient experience.14
Next Steps
If you have OAG or OHT and want to know whether Durysta could help you reach your pressure goals with fewer drops, schedule a visit with a board-certified ophthalmologist (preferably a glaucoma specialist). You can search for one by location and subspecialty in the AAO directory.15 Bring your medication list, allergy list, recent pressure readings, and past surgery or laser dates so your doctor can personalize your plan.
Urgent symptoms like sudden severe eye pain, halos, headache, or a rapid change in vision can signal an emergency—seek care right away.12 Kerbside can connect you with the right specialist for a medical education consult (not a physician–patient relationship).