Glaucoma Drainage Implant
Also known as GDI, Tube Shunt
Medical Disclaimer: Information on this page is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment.
See our Terms and Telemedicine Consent for details.

Overview
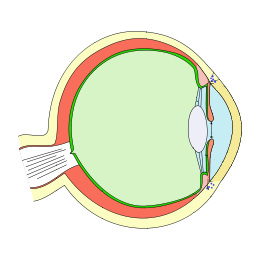
A glaucoma drainage implant (GDI) is a tiny tube attached to a thin plate that is placed on the white of the eye (sclera). The tube creates a new pathway for the eye’s fluid (aqueous humor) to leave the eye, lowering eye pressure and helping protect the optic nerve from further damage. You may also hear this called an aqueous shunt or tube shunt. 1 In general, glaucoma implants are used when medicines and laser treatments are not enough or when certain glaucoma types (like neovascular or injury-related glaucoma) make other surgeries less likely to succeed.
While a GDI cannot restore vision already lost, it can lower intraocular pressure (IOP) to slow or stop further vision loss. Your ophthalmologist will recommend this surgery based on your eye pressure, your glaucoma type, and how you responded to treatments so far. The operation is usually outpatient, and most people go home the same day with a plan for close follow-up. 2
How the Procedure Works & Options
During surgery, the plate is secured to the surface of the eye under the eyelid, and a soft tube is guided into the front of the eye (or sometimes the sulcus or pars plana, depending on lens status). The fluid drains to a reservoir over the plate where it is slowly absorbed by tissues, which reduces IOP. 3 There are two main categories of implants: valved (e.g., Ahmed) that start working right away with built-in flow restriction, and non-valved (e.g., Baerveldt, Molteno) that are temporarily tied off to prevent very low pressure until a capsule forms around the plate. 4
Your surgeon will choose device type and size based on target pressure, scarring risk, prior surgeries, and the specific glaucoma. In some eyes, the tube is covered with a patch graft (donor sclera, cornea, pericardium) to protect it. Technique and implant choice may differ if you have pediatric, uveitic, or neovascular glaucoma, or if the tube must be placed away from the cornea.
Who Is a Candidate?
GDIs are often recommended when eye drops and laser treatments have not adequately controlled IOP, or when the eye’s tissues are likely to scar after other surgeries. They are used for several glaucoma types, including congenital, neovascular, traumatic, and some complex secondary glaucomas. People with advanced damage, high IOP despite medicines, or difficulty adhering to drops may also be considered.
Other factors your doctor weighs include corneal health, prior eye operations, cataract status (sometimes combined surgery is planned), and your ability to attend frequent early follow-ups. 5 Your surgeon will discuss whether a valved or non-valved device best matches your goals and risks. 6
Am I a Candidate for a Glaucoma Drainage Implant?
Select your details to estimate suitability.
Cost and Price
Costs vary by setting (ambulatory surgery center vs. hospital), your insurance, anesthesia needs, and whether another procedure (like cataract surgery) is done at the same time. Medicare states that many medically necessary inpatient and outpatient surgeries are covered; your share depends on deductibles, coinsurance, and facility type. In outpatient hospital settings under Part B, you typically pay a percentage for the surgeon’s services and a separate copayment to the facility, subject to the Part B deductible. 8
Because coverage rules and contracted rates differ, ask your surgeon’s office for a personalized estimate and check with your insurer. They can itemize expected charges (surgeon, anesthesia, facility, implant, postoperative medicines) and clarify prior authorization steps. 7 Financial coordinators can also discuss payment plans or supplemental coverage options for your situation. 8
Benefits and Limitations
Benefits include meaningful IOP reduction when medicines and lasers are not enough and a lower chance of early scarring compared with some other filtering surgeries, especially in eyes with prior surgery or high scarring risk. Long-term randomized data (Primary Tube Versus Trabeculectomy, PTVT) show that tube shunts and trabeculectomy yield similar average IOP at 5 years, though trabeculectomy often needs fewer medications. 9 Among implants, pooled 5-year results from the Ahmed vs Baerveldt randomized trials suggest the Baerveldt device may achieve lower IOP on fewer drops but with a higher hypotony risk than Ahmed.
Limitations & risks include need for ongoing drops in some patients, transient pressure spikes (“hypertensive phase,” especially with valved devices), double vision, bleeding, infection, corneal problems, tube exposure, or need for further surgery. Careful device selection, tube positioning, and patch graft use help reduce these risks, and many issues can be managed if caught early at follow-up. Risk–benefit balance is individualized based on your eye, vision goals, and daily life. 10
Recovery and Long-Term Care
Most people go home the same day. Expect protective shield usage and prescription eye drops (antibiotic and steroid) for several weeks. Very early visits check tube position, pressure, and healing; visit frequency tapers as the eye stabilizes. For non-valved implants, your pre-op drops may continue until the tube is opened and the plate capsule matures. 11 Your team will explain activity limits (no heavy lifting, eye rubbing, or dirty water in the eye) and when to resume work, driving, or exercise. 12
Long-term, you’ll have regular pressure checks and optic nerve/visual field monitoring. Some patients still need glaucoma drops or laser touch-ups; a smaller number may need revisions (for example, if the tube becomes blocked or erodes) or additional glaucoma surgery later. Knowing the warning signs—eye pain, sudden vision change, redness, discharge, or exposure of the tube—helps you get timely care. Keep your follow-ups; they are key to staying safe and preserving vision.
Latest Research & Innovations
Large, modern trials continue to refine who benefits most from tube shunts vs. trabeculectomy. A comparative analysis of the TVT and PTVT trials found similar long-term pressure control overall, with differences in medication needs and safety profiles that guide personalized choices. Recent PTVT data also suggest that when reoperations are needed, outcomes after additional surgery are broadly similar regardless of the first procedure.
On the device side, engineers are exploring new plate materials and flow-control designs to reduce early pressure dips and late scarring, while maintaining strong pressure lowering. Early-stage innovations are being tested alongside established devices to see whether they can simplify care and improve comfort and safety over time. 13 As always, your surgeon will recommend options with the best evidence for your specific eye. 14
Recent Peer-Reviewed Research
Glaucoma Surgery: From the Tried and True to the Novel and New.
Gedde SJ, Herndon LW Jr
Intraocular lens subluxation obstructing a glaucoma tube shunt after a Valsalva maneuver: a case report.
Ha A, Nam KT, Kim S, et al.
The Rate of Failure of Trabeculectomy and Tube Shunt Surgery in Eyes with Uveitic Glaucoma and Ocular Hypertension.
Groth SL, Newcomb CW, Yang W, et al.
Next Steps
If you’re considering a glaucoma drainage implant, bring your questions about goals (target IOP), device type, anesthesia, recovery, driving/work plans, and costs. It can help to list your current eye medicines, allergies, and other health issues before your visit. 15 A trusted caregiver is often useful at early visits to help remember instructions and support eye-drop schedules. 16
Most relevant specialist: a glaucoma specialist (ophthalmologist). You can connect with the right specialist on Kerbside for a medical education consult tailored to your situation. (This is for educational guidance only and does not create a physician–patient relationship.) If you already have a surgeon, share this page at your pre-op visit to review expectations and recovery steps together.