Angle-Closure Glaucoma
also known as Closed-Angle Glaucoma, Narrow-Angle Glaucoma
Last updated July 28, 2025
Medical information on this page is for educational purposes only and is not a substitute for professional medical advice, diagnosis or treatment.
See our Terms & Conditions and Consent for Telemedicine for details.
Overview
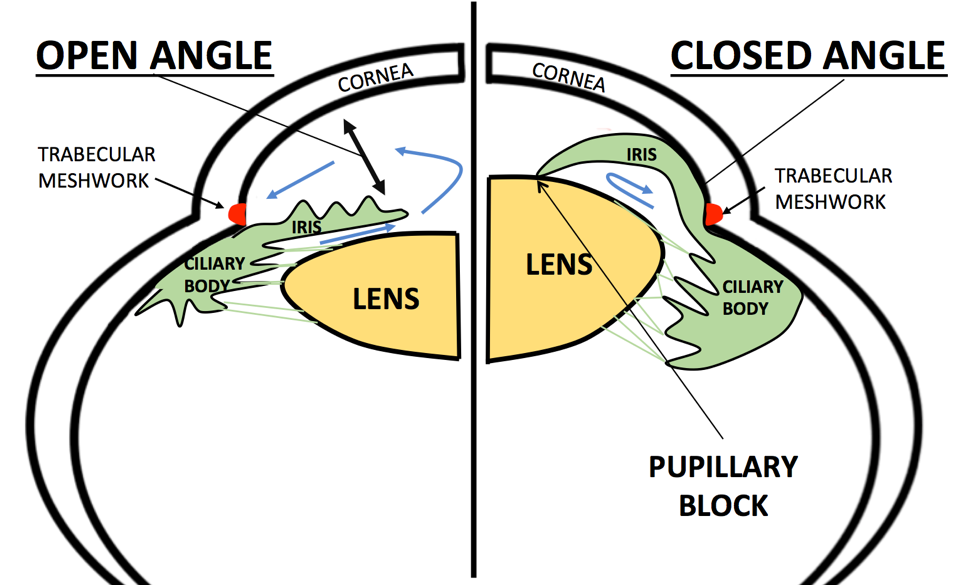
Angle-closure glaucoma (ACG)—sometimes called closed-angle or narrow-angle glaucoma—occurs when the outer edge of the iris bows forward and blocks the eye’s drainage angle. Aqueous fluid can no longer leave through the trabecular meshwork, intra-ocular pressure (IOP) rises quickly, and the optic nerve is injured if treatment is delayed.1 Although it accounts for only 10 % of glaucoma cases worldwide, ACG is responsible for a disproportionately high share of glaucoma-related blindness, especially in Asia and among older women with farsighted eyes.2 Most eyes first pass through a primary angle-closure suspect (PACS) stage, progress to primary angle closure (PAC) when pressure or peripheral anterior synechiae develop, and finally to primary angle-closure glaucoma (PACG) once optic-nerve damage is documented. Timely laser peripheral iridotomy (LPI) or lens extraction can halt this sequence and preserve sight.
Symptoms
ACG spans a spectrum from silent to dramatic:
- Chronic (creeping) closure: The angle narrows gradually; many people feel fine but slowly lose side vision and need routine gonioscopy to catch changes.3
- Intermittent (sub-acute) attacks: Transient eye pain, halos, or blurred vision in dim light that clear when pupils constrict.
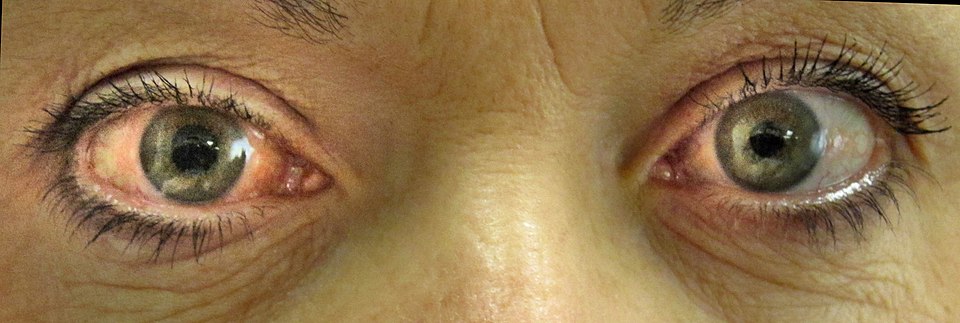
- Acute angle-closure crisis: Sudden severe ocular pain, red eye, headache, nausea/vomiting, mid-dilated unreactive pupil, and hazy cornea. IOP can exceed 50 mm Hg within hours and constitutes an ophthalmic emergency.4
Because the uninvolved eye shares the same anatomy, it is at high risk for a similar attack and should be prophylactically treated once the acute eye is stabilized.
Causes and Risk Factors
The most common mechanism is pupillary block: aqueous humor trapped behind the iris pushes it forward, sealing the angle. Contributors and risk markers include:
- Shallow anterior chamber: Typical in hyperopic eyes and with age-related lens thickening.
- Age ≥ 50 years & female sex.
- Asian or Inuit ancestry—odds of PACG are 2- to 4-fold higher.5
- Positive family history; several HLA and PLEKHA7 variants have been linked to PACG.
- Plateau iris configuration or nanophthalmos.
- Medications that dilate the pupil (e.g., anticholinergics, adrenergic agents) or environments with dim illumination.
Knowing these factors helps clinicians decide who benefits from prophylactic laser treatment.
Angle-Closure Glaucoma Risk Calculator
Enter your details in the following fields to calculate your risk
Risk Level
Recommendation
Diagnosis
A comprehensive glaucoma work-up includes:
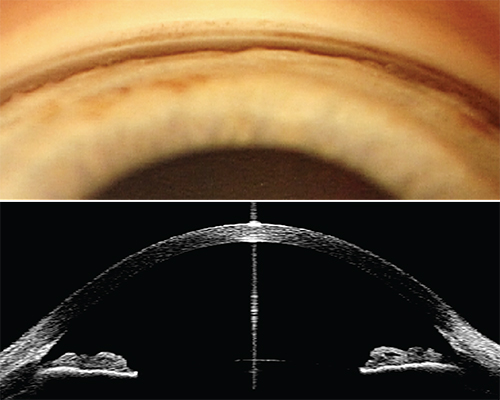
- Gonioscopy: Gold-standard assessment of the iridocorneal angle. Any trabecular meshwork not visible for >180° indicates narrow or closed angle.
- Anterior-segment OCT (AS-OCT) or ultrasound biomicroscopy to quantify angle opening distance and identify plateau iris.6
- Tonometry: Elevated IOP suggests progression from suspect to PAC or PACG.
- Optic-nerve evaluation & visual-field testing: Detect glaucomatous damage.
- Van Herick or pen-light test: Quick chair-side screening but inadequate as a stand-alone method.
Documenting angle anatomy on the day of diagnosis guides urgent versus elective intervention.
Treatment and Management
Acute attack (IOP crisis)
- Topical beta-blocker, alpha-agonist, and pilocarpine every 15 min plus oral/IV acetazolamide to lower IOP.
- Once corneal edema clears, perform laser peripheral iridotomy (LPI) to re-establish aqueous flow between chambers.7
Chronic or fellow-eye management
- Prophylactic LPI—reduces the 5-year conversion to PACG by ~75 % in high-risk eyes.
- Early cataract extraction / clear-lens exchange: Permanently deepens the angle and increasingly rivals LPI as first-line therapy in PACG with visually significant lens changes.8
- Trabeculectomy or glaucoma drainage device for eyes with residual high IOP after LPI and lens surgery.
Living with Angle-Closure Glaucoma and Prevention
You can protect your vision by:
- Routine dilated exams with gonioscopy every 1–2 years—or sooner if you have hyperopia or Asian ancestry.
- Recognizing early symptoms (halos, brow ache in dim lighting) and seeking same-day care.
- Medication adherence: Use prescribed drops exactly as directed.
- Medication review: Tell other doctors you have narrow angles before starting decongestants or certain psychiatric drugs.
- Healthy habits: Manage blood pressure, diabetes, and stay hydrated to support optic-nerve health.9
Most people maintain excellent vision when angle closure is detected and treated promptly.
Latest Research & Developments
Highlights from recent studies:
- LiGHT-ACG trial: Early LPI plus lens extraction produced better pressure control than LPI alone in symptomatic Asian eyes.
- Sustained-release bimatoprost-ring and intracameral implants are being tested to reduce drop burden after LPI.
- AI algorithms using AS-OCT images now predict which PACS eyes will progress to PAC within 18 months, enabling timely prophylaxis.
- Selective laser iridoplasty (SLIP) supplements LPI in plateau-iris syndrome and is under investigation as a primary procedure.
- Gene-therapy neuroprotection (AAV-delivered BDNF) slowed retinal-ganglion-cell death in PACG animal models.10
Recently Published in Peer-Reviewed Journals
Investigative ophthalmology & visual science
July 1, 2025
FOXP4 Variants Are Associated With Plateau Iris and Angle Closure Glaucoma.
Presley W, Wang SQ, Guan B, et al.
Investigative ophthalmology & visual science
July 1, 2025
Risk Factors for Visual Field Progression in Primary Angle Closure Disease Over 10 Years: The CUPAL Study.
Shen R, Chan PP, Ling A, et al.
Investigative ophthalmology & visual science
July 1, 2025
Glaucoma in Older Asians Aged 60 to 100 Years: Prevalence, Factors, Trends, and Projections (2024-2040).
Gupta P, Thakur S, Wong CMJ, et al.
Next Steps – See a Glaucoma Specialist Promptly
Who to see: Anyone with hyperopia, Asian ancestry, or a family history should have at least one lifelong baseline gonioscopy. If you develop acute eye pain, blurred vision, or halos, go to the nearest emergency department or on-call ophthalmologist immediately.
How to schedule: Ask for an urgent glaucoma referral; many practices reserve same-day or next-day slots for suspected angle closure. Tele-triage can help determine if an emergency visit is needed.
After evaluation and laser, keep follow-up appointments, use prescribed drops, and discuss early lens surgery if recommended. Our Kerbside platform can connect you with board-certified glaucoma specialists for second opinions and long-term care.


