Amniotic Membrane (PROKERA)
Also known as PROKERA
Medical Disclaimer: Information on this page is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment.
See our Terms and Telemedicine Consent for details.
Overview
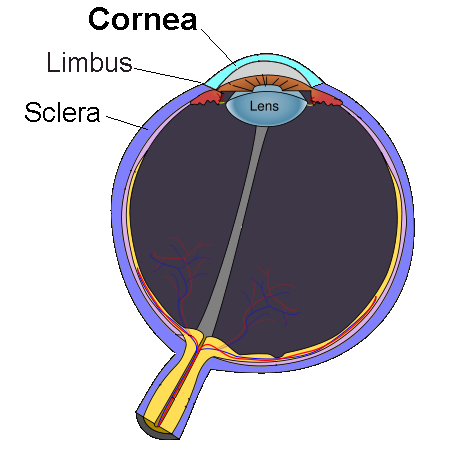
PROKERA is a sutureless, in-office amniotic membrane device used to calm inflammation, protect the cornea, and help the eye surface heal. It is often considered for moderate to severe dry eye, neurotrophic keratitis, persistent epithelial defects, and other ocular surface injuries. 1
The treatment places a thin layer of cryopreserved amniotic membrane inside a small ring system that rests on the eye like a large bandage lens and is removed after several days once healing begins.
Amniotic membrane contains natural factors that can reduce scarring and support epithelial regrowth. For many patients, this can mean faster relief of pain, light sensitivity, and blurred vision tied to a damaged corneal surface. 2 Your eye doctor will decide if PROKERA fits your condition after a slit-lamp exam and a review of prior therapies.
How the Procedure Works & Options
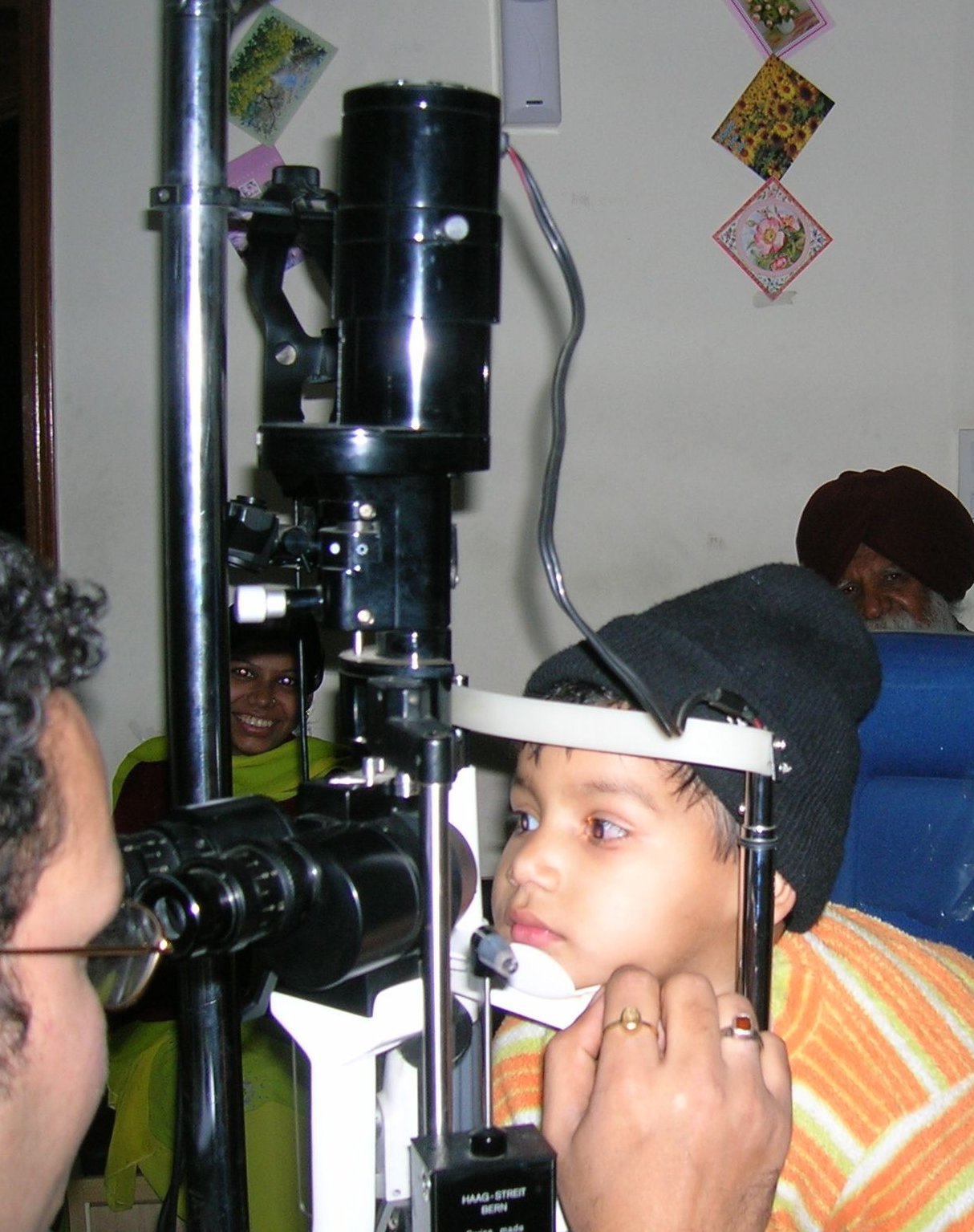
What happens in the office? After numbing drops, the clinician gently places the PROKERA ring so the cryopreserved amniotic membrane rests over the cornea. It typically stays in place for 3–7 days (sometimes longer), during which you may use prescribed drops and avoid rubbing the eye. This approach allows amniotic tissue healing without stitches. 3 PROKERA is FDA-cleared as a combination device with cryopreserved amniotic membrane specifically for ocular surface therapy. 4
Variations your doctor may consider:
- Different ring profiles (e.g., Slim, Clear) to improve comfort or visualization during care.
- Alternative forms of amniotic membrane (dehydrated discs or sutured grafts) when anatomy, exposure, or surgical plans require it.
Who Is a Candidate?
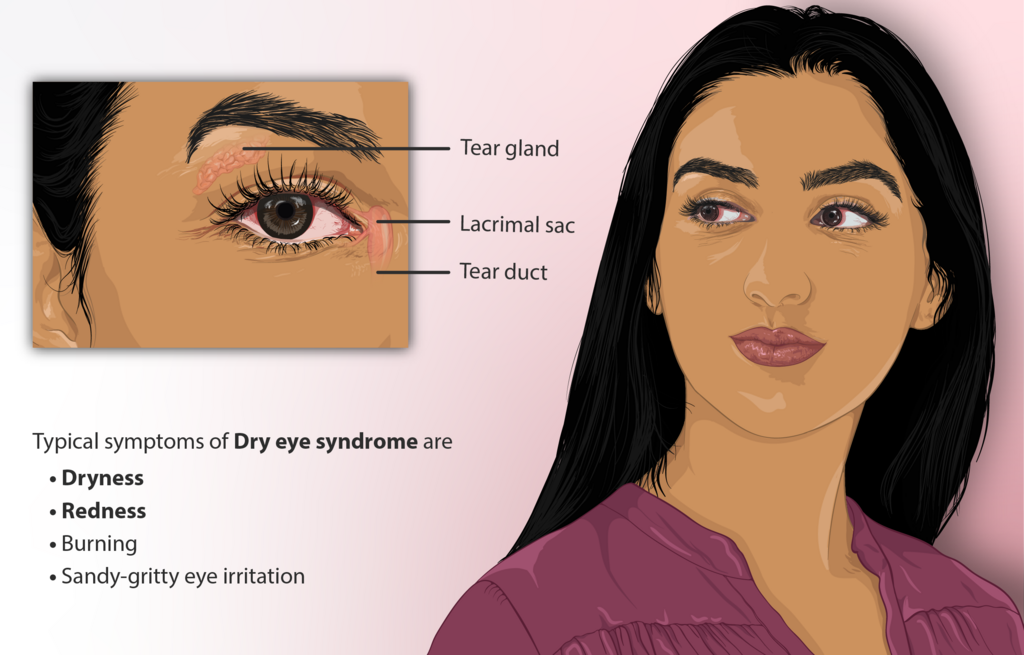
Doctors may recommend PROKERA if you have a damaged corneal surface that has not improved with standard treatments (lubricants, anti-inflammatory drops, punctal plugs, moisture goggles, or bandage contact lenses). This includes moderate to severe dry eye with epithelial staining, exposure-related surface breakdown, or neuropathic and neurotrophic conditions. 5 It can also be used in certain cases of noninfectious keratitis or after surface trauma to protect healing tissue.
You may not be a candidate if you have active, uncontrolled eye infection, severe eyelid malposition that prevents device retention, or hypersensitivity to any component. Careful screening and cultures may be done when infection is suspected, since treatment choices differ for infectious keratitis. 6 Your clinician will discuss relative benefits versus other options for your specific diagnosis.
Educational Candidacy Score for PROKERA
Select your details to estimate suitability.
Cost and Price
Costs vary by clinic and insurance. In the United States, coverage policies for sutureless placement of amniotic membrane on the ocular surface are typically determined by local Medicare contractors and private insurers; they often require documented medical necessity and appropriate diagnosis codes. Some jurisdictions have published Local Coverage Determinations (LCDs) or related guidance describing indications, frequency limits, and documentation expectations.
Out-of-pocket amounts depend on deductibles, coinsurance, and whether the service is in-network. Ask your clinic for a personalized estimate before scheduling, and bring your prior treatment history (drops, plugs, lenses) since documentation of failed conservative therapy is often required for reimbursement. If insurance does not cover the procedure in your case, clinics may offer payment plans. 8
Benefits and Limitations
Potential benefits include faster epithelial healing, reduced inflammation and scarring, and improved comfort and vision as the ocular surface stabilizes. These effects have been reported across a range of corneal disorders, including persistent epithelial defects and ulcers, with amniotic membrane acting as a biologic bandage. 9 Because PROKERA is self-retained, it avoids sutures and can be placed in the office, which may improve access and convenience for appropriate patients.
Limitations and risks include temporary blur, foreign-body sensation from the ring, device intolerance, membrane displacement, or, rarely, infection. Clinicians monitor closely and remove the device if problems arise. Case literature describes rare instances of microbial keratitis in high-risk eyes, underscoring the need for careful selection and follow-up. 10 Discuss alternatives (e.g., bandage lenses, serum tears, scleral lenses, or sutured grafts) to decide what fits your goals.
Recovery and Long-Term Care
Most patients wear the device for several days. During that time, plan for light activity, avoid eye rubbing, and use prescribed drops as directed. Your vision may be temporarily blurry while the ring is in place; this clears after removal. 12 The doctor will examine the epithelium to confirm closure and may recommend ongoing surface therapy (lubricants, lid hygiene, anti-inflammatory medications) to maintain gains.
Long-term care focuses on controlling the underlying cause (e.g., evaporative dry eye, exposure, or nerve dysfunction) and protecting the cornea. Some patients need repeated treatments if the surface breaks down again; others transition to scleral lenses or immunomodulatory drops to keep the eye stable. 11 Close follow-up is key to catch recurrence early and adjust the plan.
Latest Research & Innovations
Recent clinical research has explored how cryopreserved amniotic membrane may help regenerate corneal nerves and improve sensitivity in dry eye disease, with randomized data showing structural and symptomatic benefits compared with conventional therapy. 13 Emerging retrospective series also report outcomes using PROKERA across a spectrum of ocular surface diseases, helping refine which patients respond best and how long devices should remain in place.
Ongoing investigations compare cryopreserved versus dehydrated amniotic membranes, study pre-surgical use to stabilize the surface before cataract or refractive surgery, and evaluate objective measures like corneal staining, nerve imaging, and patient-reported comfort scores. These efforts aim to personalize treatment and reduce time to healing. 14 Talk with your specialist about how current evidence applies to your diagnosis and goals.
Recent Peer-Reviewed Research
Utilization Patterns and Costs of Ocular Amniotic Membrane Grafts in the Medicare Population.
Vail D, Nudleman E, Abdeljaber L, et al.
Topical Cenegermin for Pediatric Neurotrophic Keratopathy: A Review of the Literature and Systematic Review.
Bakr M, Eleiwa TK, Saeed HN, et al.
Prospective consecutive case series of patients with neurotrophic keratopathy associated with unilateral total limbal stem cell deficiency caused by severe ocular surface burns.
Figueiredo GS, Stefanache T, Baylis OJ, et al.
Next Steps
If your eyes are painful, light-sensitive, or not improving on standard therapy, schedule a visit with a cornea specialist (ophthalmologist). They can examine your surface, explain options like PROKERA, and map a plan that addresses the cause of damage. You can search for a board-certified ophthalmologist by location. 15
To prepare, note your symptoms, triggers, and what you have already tried; learn the basics of dry eye and corneal surface disease to make the most of your appointment. 16
Kerbside helps you connect with the right specialist for an education-focused consult. This is not a physician–patient relationship or a substitute for in-person care, but it can help you understand choices and next steps.